Search Results
Results for: 'Equilibrium Constant'
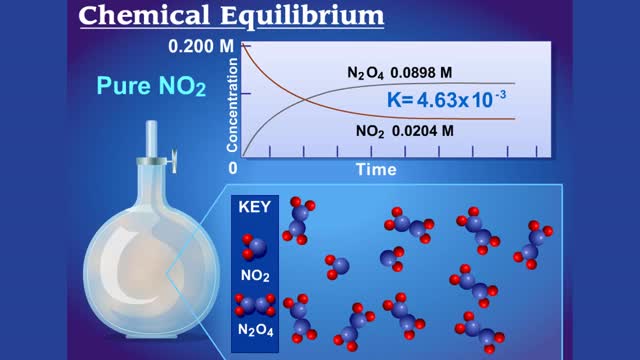
Chemical Equilibrium between N2O4 (colorless gas) and NO2 (brown gas)
By: HWC, Views: 5967
For a system at equilibrium: ◆ both forward and reverse reactions are occurring simultaneously ◆ rate of forward reaction must equal rate of reverse reaction OR Rate forward = Rate reverse ◆ concentrations of reactants and products remain constant with time the equilibrium positio...
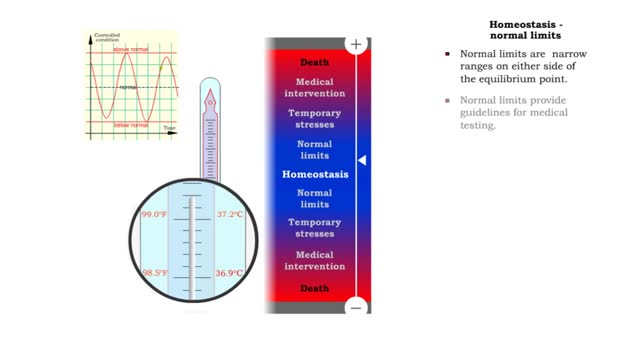
Homeostasis (range of body conditions, normal limits, mild stresses & severe stresses)
By: HWC, Views: 6604
Homeostasis: • Provides relative stability of the internal environment. • Results from constant adjustments. • Regulated by regulatory processes. • Requires system interplay. • Normal limits. • Temporary stresses. • Disruptions requiring medical intervention. • D...
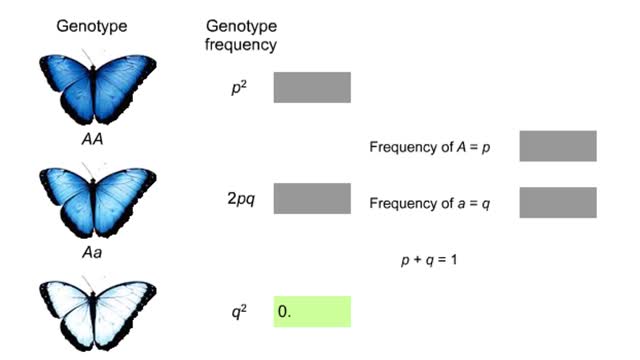
How to find out if a population is evolving?
By: HWC, Views: 3602
Imagine a butterfly population with a pair of alleles that influence wing color as shown. We will represent the frequency of the dominant allele as p and the recessive allele as q. The Hardy-Weinberg rule describes what happens if a population is at genetic equilibrium—if it is not evolving...
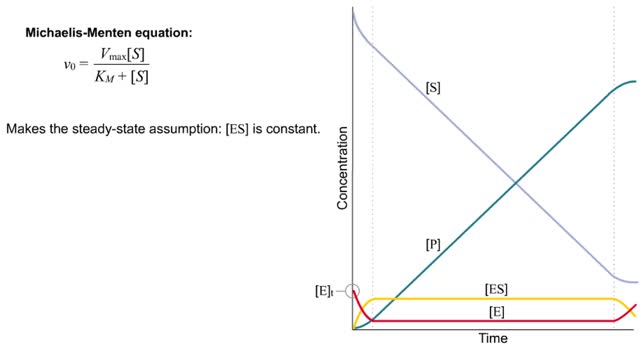
Michaelis–Menten equation & Kinetic parameters
By: HWC, Views: 6390
The Michaelis–Menten equation is the rate equation for a one-substrate enzyme-catalyzed reaction. This equation relates the initial reaction rate (v0), the maximum reaction rate (Vmax), and the initial substrate concentration [S] through the Michaelis constant KM—a measure of the substrat...
Membrane Protein and Facilitated Transport (Passive Vs Active)
By: HWC, Views: 6261
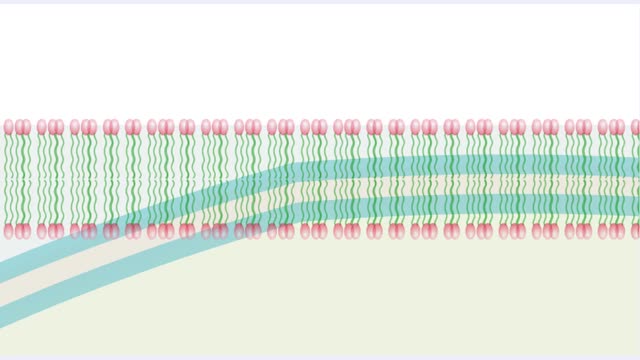
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins span the membrane, with hydrophobic amino acids interacting with the lipid bilayer and hy...
By: HWC, Views: 6141
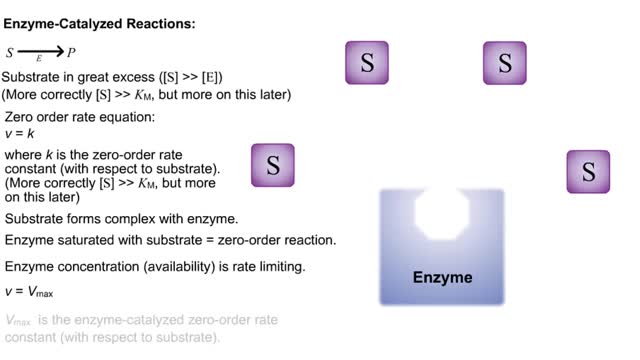
S P Substrate in great excess ([S] -- [E]) (More correctly [S] -- KM, but more on this later) Zero order rate equation: v = k where k is the zero-order rate constant (with respect to substrate). (More correctly [S] -- KM, but more on this later) Substrate forms complex with enzyme. ...
Import of Dietary Glucose from Intestines to Bloodstream
By: HWC, Views: 6110
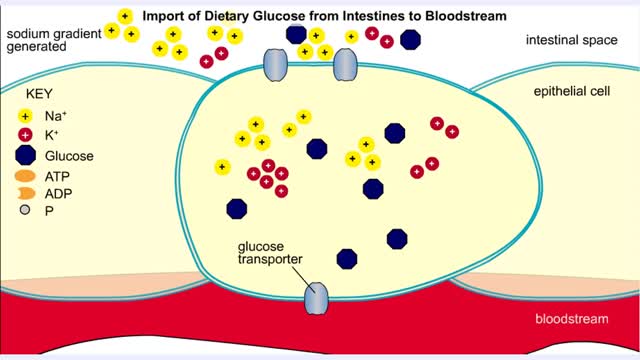
• Membranes have hydrophobic interiors. which resist the passage of hydrophilic compounds and ions. • However. transporter membrane proteins facilitate the passage of these molecules. • Passive transporters accelerate diffusion of molecules towards equilibrium (decrease a concentrat...
Molecules, Membrane Permeability and Structure
By: HWC, Views: 6149
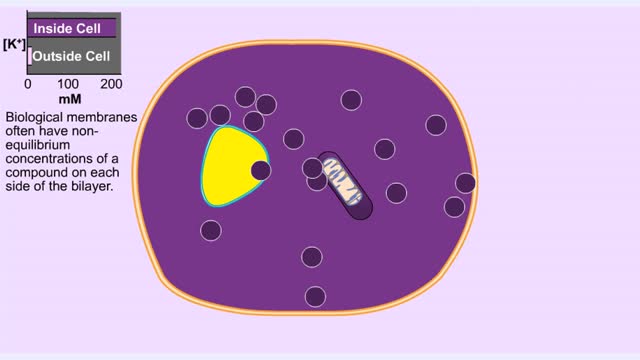
Organisms are not isolated system at equilibrium and need to intake nutrients and electrolytes as remove wastes. Similarly Cells within an organism must also exchange compound by passing them through membrane. The permeability of a membrane is the rate of passive diffusion of molecules th...
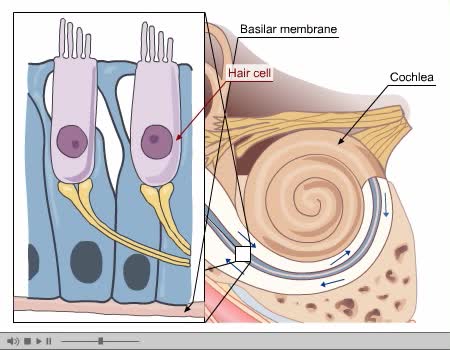
By: Administrator, Views: 9911
Process of Hearing Sound waves are directed to the eardrum, causing it to vibrate. These vibrations move the three small bones of the middle ear (malleus, incus, and stapes). Movement of stapes at oval window sets up pressure waves in the perilymph and endolymph. Process of Hearing The wav...
Advertisement